RadCalc QA software verifies non-standard treatment plans for HDR brachytherapy
Medical physicists at University Hospitals Birmingham in the UK rely on LAP’s RadCalc QA secondary check software to support their high-dose-rate brachytherapy programme.
Find the original article on the Physics World website.
Independent patient QA and secondary dose calculations are key to safe, consistent and efficient radiation delivery within the high-dose-rate (HDR) brachytherapy programme at University Hospitals Birmingham NHS Foundation Trust, a healthcare network serving the West Midlands region of the UK. With this in mind, the Birmingham radiation oncology team has, for the past decade, relied on LAP’s RadCalc QA secondary check software – a suite of widely deployed QA tools that provides medical physicists and dosimetrists with fully automated and independent dosimetric verification of their radiotherapy treatment planning systems (TPS) – for the immediate validation of its brachytherapy plans.
For context, HDR brachytherapy involves the clinical application of radioactive isotopes to deliver therapeutic radiation to internal or superficial tumours – a targeted procedure that allows a higher dose of radiation to the tumour site before or after surgery. In the case of interstitial delivery, the radioactive source (Ir-192, for example) is placed directly into the tumour target, while contact brachytherapy requires placement of the source in a space adjacent to the target tissue (in an internal cavity, for example, or externally on the skin). Either way, HDR brachytherapy is a highly conformal radiation treatment that requires precise planning and verification to avoid collateral damage to healthy tissues and adjacent organs at risk (OARs) – including the bowel and the bladder in the case of HDR treatment of gynaecological cancers.
“The first step [in HDR brachytherapy] is to insert empty catheters or applicators into the patient for delivery of the source from the treatment unit to the tumour,” explains Ruth Wyatt, lead physicist for brachytherapy at University Hospitals Birmingham. “Everything happens in one session: the applicators or catheters are inserted; scans are taken; the treatment is planned, checked and delivered. All of which means that speed and workflow efficiency are very important.”
Streamlined checks, workflow efficiency
Within University Hospitals Birmingham, HDR brachytherapy is currently used to treat around 100 patients each year. The programme is geared exclusively for gynaecological indications, although a roadmap for the clinical roll-out of HDR prostate brachytherapy is in place, with the start date dependent on staff recruitment and specialist training.
“We use RadCalc for independent dose-calculation checks of any non-standard plans generated by our Oncentra Brachy TPS [from Elekta],” says Wyatt. The RadCalc software installation also supports independent patient QA more broadly across Birmingham’s external-beam radiotherapy programme, including secondary dose checks on the CyberKnife stereotactic treatment system as well as dose verification of electron-beam therapy for the treatment of superficial tumours.
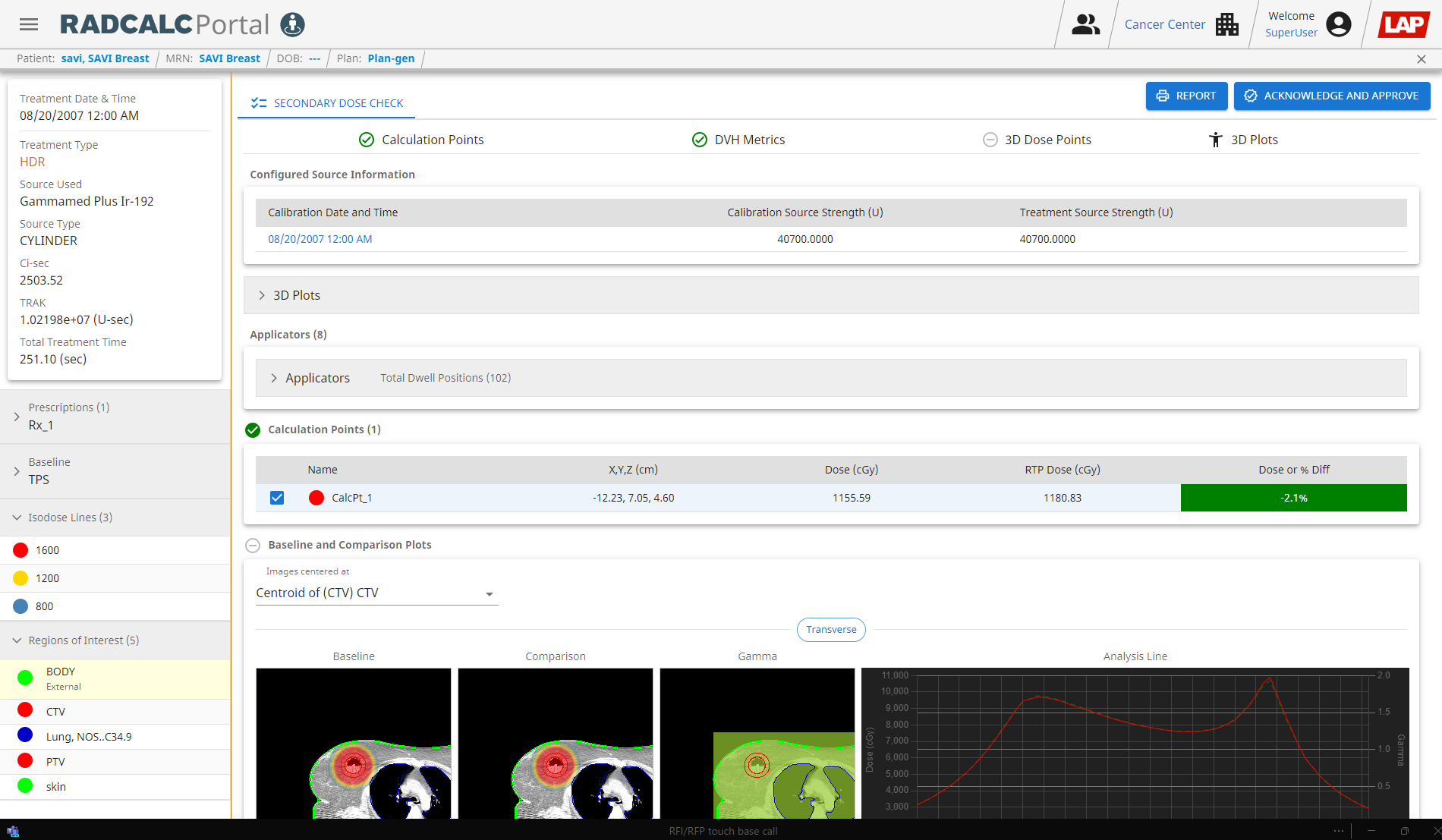
In daily clinical practice, RadCalc provides an independent check of the standard “Point A” dose points entered for each HDR brachytherapy treatment plan; also at points 5 mm lateral to the ovoid applicators (which deliver the radioactive source to the tumour) for all non-standard treatment plans. “All differences have been well within 1%,” says Wyatt. “In some cases, we also check dose at multiple points around the high-risk clinical target volume and differences are generally <0.5%.”
At a headline level, the upsides of RadCalc for Wyatt and her medical physics colleagues are clear to see. “The main benefit is the immediacy of those secondary-check results – essential for preventing errors in the planned delivery of HDR brachytherapy,” she notes. “We’ve set up a DICOM ‘listener’ to allow the export of treatment plans from the TPS followed by direct import into RadCalc. This means the brachytherapy QA workflow is extremely efficient and, by extension, so too is our patient throughput.”
The QA roadmap
Notwithstanding its importance for daily patient QA, RadCalc was also instrumental in the original set-up and commissioning of Birmingham’s Oncentra Brachy TPS (with RadCalc dose calculations for the Ir-192 HDR Flexisource based on the AAPM Task Group 43 brachytherapy protocol). “We carried out tests by creating a number of plans using single or multiple tandem and ovoid applicators in our TPS,” explains Wyatt. “The standard ‘Point A’ dose points were entered for each plan, along with several other test points.”
In this way, RadCalc automatically calculates the dose at each dose point based on the source activity exported from the TPS, displaying the result alongside the dose exported from the TPS (as well as the dose difference). “We found differences mainly <0.2%, except for positions very close to a source [inside an applicator], where they are of no clinical interest,” says Wyatt. “We also carried out independent manual checks for some plans. The dose differences here were slightly larger for plans containing both tandem and ovoid applicators, because the anisotropy of the dose distribution around the source was not taken into account in the manual calculations.”
As for the clinical roadmap at Birmingham, Wyatt and her colleagues also used RadCalc to independently validate dose calculations for ring-type applicators before they were brought into clinical use. What’s more, the team has road-tested a demonstrator version of RadCalc including the software’s 3D dose-volume functionality, exporting several clinical plans and their structure sets as part of an in-house feasibility study.
With the 3D dose calculations, RadCalc displays the dose-volume histogram (DVH) parameters exported from the TPS alongside its own independently calculated DVH parameters (although the RadCalc value imported directly from the TPS may differ slightly as it depends on the size of the dose grid selected). In addition, the ability to calculate a 3D gamma analysis is a valuable tool for plan evaluation.
“All RadCalc-calculated DVH doses for our clinical plans were within 2.0% of the TPS values,” concludes Wyatt. “Alongside the DVH comparison, the ability to send the planning CT images to overlay the critical structures also allows us to see the isodose lines within the anatomy.”
Further reading
- RadCalc’s Monte Carlo capability streamlines and automates 3D dose-volume verification
- LAP’s RadCalc software ensures independent QA for Gamma Knife Perfexion treatment planning